
Advanced oxidation processes for the removal of contaminants of…
W
ater quality can be affected by contamination due to microorganisms, trace metals, toxic chemical compounds, changes in salinity, temperature or acidity. All this contamination is removed in water treatment plants, ensuring the required quality of the water for each purpose following the correspondent legislation. However, in recent years, there has been an increase in global concern about water pollution and especially about the rise of contaminants of emerging concern (CECs) [1]. Contaminants of emerging concern can be defined as organic micropollutants, natural or synthetic, that present potential risks to health and the environment and are not, or are just beginning to be, regulated by current legislation [2]. Within this group of contaminants, we can find everyday products such as pharmaceuticals and personal care products, surfactants, plasticizers or industrial additives. These pollutants can be found in urban water and effluents from wastewater treatment plants mainly originated in industries, hospitals, pharmaceutical factories and processes related to livestock and agriculture [3].
The main problem associated with emerging pollutants is that they are refractory compounds whose removal is not completely guaranteed with biological treatments in wastewater treatment plants. Once these pollutants and their metabolites reach aquatic ecosystems, they present toxic effects and bioaccumulation. Furthermore, the effect of some of these contaminants on human health is not fully understood, presenting a risk if they reach the human body [4]. As a consequence of their refractory behaviour, it is necessary to use alternative methods, capable of producing changes in their structure and degrade them, such as Advanced Oxidation Processes (AOPs) [1].

The concept of advanced oxidation processes was first defined by Glaze et al. in 1987 as processes involving the production and utilization of highly reactive transient species, especially the hydroxyl radical [6]. This species presents a great oxidizing power as a consequence of its high redox potential (2.80 V). The hydroxyl radicals generated by the process are responsible for oxidizing the contaminants of emerging concern present in the water by different pathways, in many cases achieving total mineralization (i.e., full degradation into carbon dioxide and water). There are many different advanced oxidation processes that can generate hydroxyl radicals by the action of light and/or chemical agents [7].
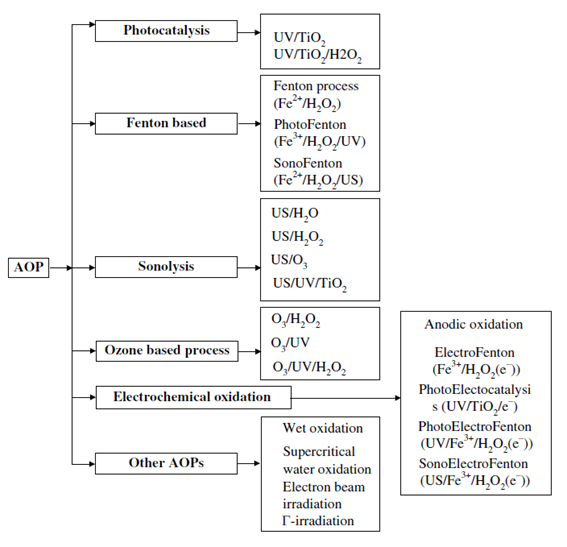
Recently, heterogeneous photocatalysis has become one of the most studied AOPs. This process is based on the use of a solid semiconductor that is activated by the action of light. When the catalyst is irradiated it absorbs light so electrons “jump” from the valence band to the conduction band, creating an electron-hole pair (e–/h+). Of these two formed species, the hole can react with water, oxidising it to produce the desired hydroxyl radicals. One key factor of photocatalysis is the wavelength of the light used. The energy of the light has to be enough to overcome the energy bandgap of the photocatalyst. Although several semiconductors can be used as photocatalysts, titanium dioxide is the most widely employed [7]. However, with a bandgap of 3.2 eV, it requires ultraviolet light for its activation, which requires a lot of energy. Hence there is a lot of research working for being able to carry out photocatalysis with solar radiation, aiming for a more sustainable process [8].

AOPs are usually limited by the rate of radical production and not so much by the degradation reaction of contaminants. An important role in the rate is played by the presence of radical scavengers, mainly dissolved organic matter, that can have a much higher concentration than CECs, blocking their degradation. Therefore, this process must be placed at the end of the water treatment, when other contaminants that can inhibit the degradation have already been removed.
Summing up, CECs present a risk to the environment and human health so they are starting to be regulated and need to be removed in WWTPs. Advanced oxidation processes can be the solution to this problem, in which photocatalysis can play an important role, especially if it can be achieved using solar light. This process could be installed in the tertiary treatment of wastewater treatment to ensure the removal of these pollutants.
References
[1] M. Salimi et al., “Contaminants of emerging concern: a review of new approach in AOP technologies,” Environ. Monit. Assess., vol. 189, no. 8, 2017.
[2] T. Deblonde, C. Cossu-Leguille, and P. Hartemann, “Emerging pollutants in wastewater: A review of the literature,” Int. J. Hyg. Environ. Health, vol. 214, no. 6, pp. 442–448, 2011.
[3] A. Pal, K. Y. H. Gin, A. Y. C. Lin, and M. Reinhard, “Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects,” Sci. Total Environ., vol. 408, no. 24, pp. 6062–6069, 2010.
[4] S. A. Fast, V. G. Gude, D. D. Truax, J. Martin, and B. S. Magbanua, “A Critical Evaluation of Advanced Oxidation Processes for Emerging Contaminants Removal,” Environ. Process., vol. 4, no. 1, pp. 283–302, 2017.
[5] https://www.slu.se/en/ew-news/2019/6/cec-policy-brief-english/
[6] Quiroz, M. A.; Bandala, E. R.; Martínez-Huitle, C.A. Advanced Oxidation Processes (AOPs) for removal of pesticides from aqueous media. In: Pesticides- Formulations, Effects, Fate. Editor: Margarita Stoytcheva. InTech, 2011.
[7] G. Divyapriya, I. M. Nambi, and J. Senthilnathan, “Nanocatalysts in fenton based advanced oxidation process for water and wastewater treatment,” J. Bionanoscience, vol. 10, no. 5, pp. 356–368, 2016.
[8] C. Sordo, R. Van Grieken, J. Marugán, and P. Fernández-Ibáñez, “Solar photocatalytic disinfection with immobilised TiO2 at pilot-plant scale,” Water Sci. Technol., vol. 61, no. 2, pp. 507–512, 2010.









