Blog
Blog
Fat, oil and grease removal: the forgotten step during…
The energy efficiency in the water sector is gaining more and more importance, pursuing net zero targets for energy and carbon footprint neutrality [1]. Most wastewater treatment plants (WWTP) follow a traditional model consisting of pre-treatment, primary settling, biological (activated sludge) reactors and secondary settlers [2]. The pre-treatment usually comprises several steps to remove coarse materials such as wipes (>1 cm), high density materials such as sands and low density compounds such as fat, oil and grease (FOG). FOG must be removed during the pre-treatment as they can cause blockages in the next-steps infrastructure, impede adequate clarification and affect the activity of microorganisms in biological units [3], [4].

FOG removal is normally achieved through flotation processes, which are based on increasing the density difference between continuous and dispersed phases. This separation is achieved adding air to the wastewater, promoting formation of air-FOG agglomerates that float to the surface where they can be easily removed [5]. Induced air flotation (IAF) systems use turbines to induce the generation of small air bubbles that enable the separation by density. These systems present some advantages as a high separation efficiency, high loading-rates and short retention times and a low carbon footprint [6].
However, the operation of pre-treatment systems for the removal of FOG are not often optimized. The most important parameter in the design and operation of FOG removal systems is the ratio between air and FOG. On one hand, if the air/FOG ratio is low, the FOG removal won’t be effective. On the other hand, if too much air is induced, the energy consumption of the system would be high and the system won’t be efficient. However, in practice, most FOG removal systems are operated with fixed parameters, not considering the variations in the flowrate or the organic loading rate of the wastewater inlet. Some challenges limit the implementation of control strategies, such as the lack of quantification methods for FOG content in wastewater and the lack of online monitoring systems [7], [8].
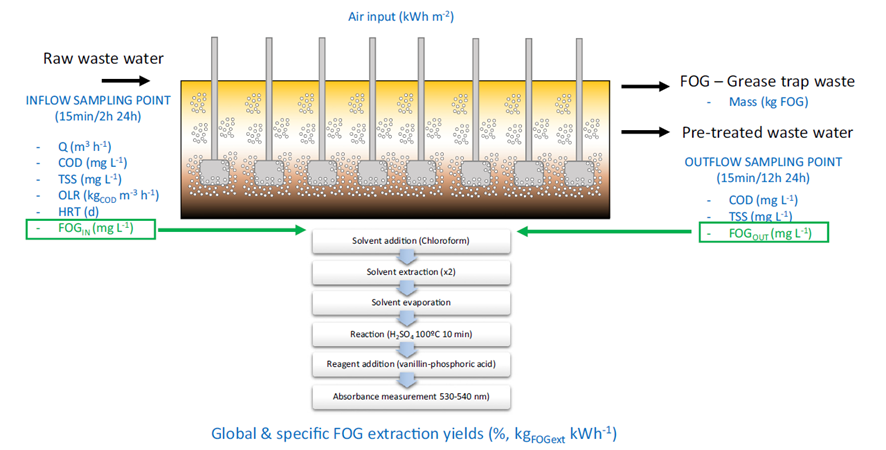
FOG determination and quantification is normally carried out using infrared spectroscopy. However, this technology is expensive and is usually not available on WWTP laboratories, making difficult for operators to collect the information required to optimize FOG removal systems [9]. However, inspired by the methodology for lipid quantification in biological systems, a simple methodology based on UV spectroscopy has recently been developed, showing comparable results to traditional IR methods, and being able to be easily implemented in any WWTP laboratory [7].
The implementation of quantification protocols, enable the optimization of FOG removal. The process can be optimized to ensure the air/FOG ratio is always optimum depending on the inlet variables such as the organic loading rate or the flowrate income. This optimization can make a huge difference, being able to reduce the energy consumption of FOG removal systems up to 40%. Furthermore, this optimization maximises the FOG recovery. FOG can be then easily conditioned with an alkaline treatment [10] to be used as a co-substrate in anaerobic digesters to increase the biogas produced and the energy self-sufficiency of WWTP.
References
[1] A. Soares, “Wastewater treatment in 2050: Challenges ahead and future vision in a European context,” Environ. Sci. Ecotechnology, vol. 2, p. 100030, Apr. 2020, doi: 10.1016/j.ese.2020.100030.
[2] J. Palatsi, F. Ripoll, A. Benzal, M. Pijuan, and M. S. Romero-güiza, “Enhancement of biological nutrient removal process with advanced process control tools in full-scale wastewater treatment plant,” Water Res., vol. 200, p. 117212, 2021, doi: 10.1016/j.watres.2021.117212.
[3] M. Usman, E. S. Salama, M. Arif, B. H. Jeon, and X. Li, “Determination of the inhibitory concentration level of fat, oil, and grease (FOG) towards bacterial and archaeal communities in anaerobic digestion,” Renew. Sustain. Energy Rev., vol. 131, no. July, p. 110032, 2020, doi: 10.1016/j.rser.2020.110032.
[4] C. Gurd, R. Villa, and B. Jefferson, “Understanding why fat, oil and grease (FOG) bioremediation can be unsuccessful,” J. Environ. Manage., vol. 267, no. October 2019, p. 110647, 2020, doi: 10.1016/j.jenvman.2020.110647.
[5] P. Painmanakul, P. Sastaravet, S. Lersjintanakarn, and S. Khaodhiar, “Effect of bubble hydrodynamic and chemical dosage on treatment of oily wastewater by Induced Air Flotation (IAF) process,” Chem. Eng. Res. Des., vol. 88, no. 5–6, pp. 693–702, 2010, doi: 10.1016/j.cherd.2009.10.009.
[6] W. H. Zhang, I. Kaur, W. Zhang, J. Shen, and Y. Ni, “Recovery of manool from evaporator condensate by induced air flotation in a kraft pulp mill based integrated biorefinery,” Sep. Purif. Technol., vol. 188, pp. 508–511, 2017, doi: 10.1016/j.seppur.2017.07.063.
[7] M. Romero-Güiza, R. Asiain-Mira, M. Alves, and J. Palatsi, “Induced air flotation for fat, oil, and grease recovery in urban wastewater: A proposed methodology for system optimization and case study,” J. Water Process Eng., vol. 50, no. March, 2022, doi: 10.1016/j.jwpe.2022.103201.
[8] N. Shammas, Flotation Technology, no. June 2010. Totowa, NJ: Humana Press, 2010.
[9] M. V. Melo, G. L. Sant’Anna, and G. Massarani, “Flotation techniques for oily water treatment,” Environ. Technol. (United Kingdom), vol. 24, no. 7, pp. 867–876, 2003, doi: 10.1080/09593330309385623.
[10] M. S. Romero-Güiza, J. Palatsi, X. Tomas, P. Icaran, F. Rogalla, and V. M. Monsalvo, “Anaerobic co-digestion of alkaline pre-treated grease trap waste: Laboratory-scale research to full-scale implementation,” Process Saf. Environ. Prot., vol. 149, pp. 958–966, 2021, doi: 10.1016/j.psep.2021.03.043.